
Luxembourg – the land of roses

Still life by Abraham Mignon
We have just been to Luxembourg, which is also called the land of roses. Therefore, today my scientific contribution to a rose, which, however, is no longer in bloom but is in a painting by Abraham Mignon (a Dutch painter 1640-1679). This yellow rose in the centre of the painting looks very faded, monotonous and not at all fitting in contrast to the other flowers in this lush bouquet.

Detail with pale yellow (or now ochre) rose.
I have now learned that this is due to chemistry, and not because Mr Mignon painted this rose so inconspicuously. On the contrary A. Mignon used a pigment for this rose – orpiment – which at that time conjured up the most beautiful shades of yellow on canvas. Before the invention of chrome yellow, orpiment was the most luminous yellow known in painting (already since ancient times). Orpiment, is also known under the name auric pigment or arsenic blend, as well as under its chemical name arsenic (III) sulfide, and is an arsenic-sulfur mineral.

The pigment that produced the most beautiful yellow-orange colors in painting was orpiment or auric pigment.
But what happened to the beautiful yellow of the orpiment of this rose?

Mimetite no longer “glows” yellow
An analysis of this one rose in the Rijksmuseum in Amsterdam by non-invasive techniques such as X-ray fluorescence imaging and X-ray powder diffraction (SN: 10/1/21), revealed the remaining traces of arsenic, lead, calcium and other chemical elements in the paint layers that Mignon may very well have carefully layered the paint to create an almost three-dimensional rose of light and shadow. In addition, however, they found crystals known as mimetite and schultenite.
These are the result of a series of chemical reactions. First, the reaction of orpiment with light and oxygen created the very reactive arsenolite. This arsenolite then found its way to and chemically reacted with an underlying layer of lead white paint, and mimetite (a lead arsenate) and schultenite (lead hydrogen arsenate) were formed. The crystals of these two substances do not have the bright colour of the orpiment, but are colourless and flatten the appearance of the flower.

Many paintings from the Baroque period have a “blue cast” because the yellow faded.
Other paintings whose artists used this pigment also suffer from this decrease in the intensity of the yellow. This is the reason that in many old landscape paintings the trees have turned blue. In fact, the old masters, in the absence of a beautiful green pigment, often mixed the colour green from orpiment varnish and a blue pigment. With the “fading” of the yellow orpiment, only the blue tones remained……
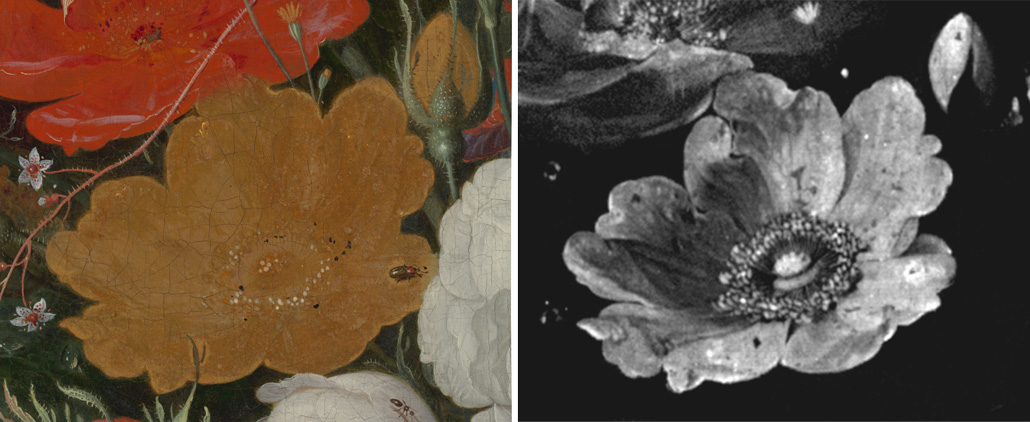
This yellow rose (left) is shown as it appears to the naked eye today in the painting Still Life with Flowers and a Watch. X-ray fluorescence imaging reveals a more masterful ghost of the past. The image (right) shows the elemental distribution of arsenic that remains in the rose. Originally painted in an arsenic sulfide–based bright yellow pigment, chemical reactions with light and with other paint layers dulled the flower’s appearance over time.N. De Keyser et al/Science Advances 2022
Science cannot turn back the clock on chemical transformation to restore the rose to its former glory – that is reversible. But digital reconstructions, as applied at the Rijksmuseum in Amsterdam, can reveal the faded elements, providing a “ghostly” glimpse into a painting’s past beauty.
Citations
N. De Keyser et al. Reviving degraded colours of yellow flowers in 17th century still life paintings with macro- and microscale chemical imaging. Science Advances. Published online June 8, 2022. DOI: 10.1126/sciadv.abm2106.
